what happened when the splint was brought to the mouth of the first bottle of gas collected
4: The Backdrop of Oxygen Gas (Experiment)
- Folio ID
- 95809
- To generate (and collect) oxygen gas via the decomposition of hydrogen peroxide.
- To investigate the backdrop of oxygen, peculiarly every bit an amanuensis of combustion.
Oxygen is 1 of the most abundant elements on this planet. Our temper is 21% free elemental oxygen. Oxygen is too extensively combined in compounds in the earths crust, such as h2o (89%) and in mineral oxides. Even the human body is 65% oxygen by mass.
Free elemental oxygen occurs naturally as a gas in the class of diatomic molecules, \(\ce{O2}\) (g). Oxygen exhibits many unique physical and chemical properties. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in h2o. In fact, the latter 2 properties greatly facilitate the drove of oxygen in this lab. Among the unique chemical properties of oxygen are its ability to back up respiration in plants and animals, and its power to support combustion.
In this lab, oxygen volition be generated as a product of the decomposition of hydrogen peroxide. A catalyst is used to speed up the charge per unit of the decomposition reaction, which would otherwise be besides dull to use every bit a source of oxygen. The catalyst does not get consumed by the reaction, and can be nerveless for re-use once the reaction is complete. The particular goad used in this lab is manganese(IV) oxide.
Generating Oxygen Gas:
\[\ce{two H2O2 (aq) ->[goad] two H2o (fifty) + O2 (g)}\]
\[\text{hydrogen peroxide} \ce{->} \text{water} + \text{oxygen}\]
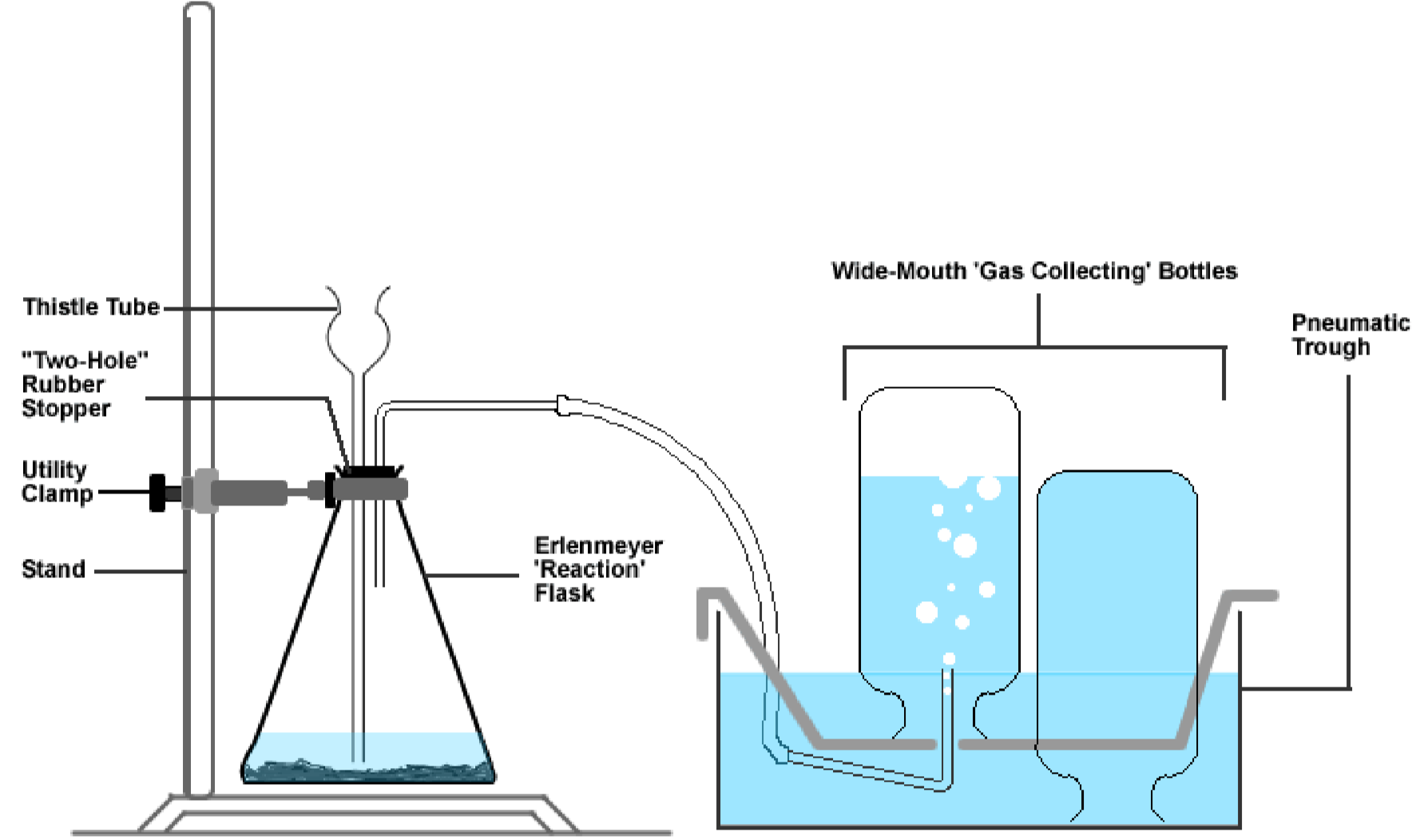
The oxygen gas produced will be nerveless in bottles by a method known as the downward displacement of water (meet effigy 1). One time collected, several tests will be performed in social club to investigate the role of oxygen in several combustion reactions.
A combustion reaction is commonly referred to every bit "burning". During a combustion reaction, oxygen reacts chemically with the substance beingness burned. Note that since our atmosphere is roughly 21% oxygen, many substances readily burn down in air. Both oxygen and the substance being burned (the reactants) are consumed during the combustion reaction, while new substances (the products) and heat energy are generated. Since oestrus is produced, this is an exothermic reaction.
Combustion Reactions:
\[\text{Substance being burned} + \text{Oxygen} \ce{->} \text{Products} + \text{Rut}\]
The actual products of a combustion reaction depend on what substance is burned and how much oxygen is present. In general, however, when a pure element burns in oxygen the product is chosen an oxide. An oxide is a chemical compound containing both the element and oxygen chemically combined together.
Some examples of element combustion are shown below. Several such reactions will be performed using the oxygen gas collected in this lab.
Combustion of an Chemical element:
\[\text{Element} + \text{Oxygen} \ce{->} \text{Oxide of Chemical element} + \text{Rut}\]
\[\ce{C(s) + O2(chiliad) -> CO2(thou) + Oestrus}\]
\[\text{carbon} + \text{oxygen} \ce{->} \text{carbon dioxide} + \text{heat}\]
\[\ce{2Hg(fifty) + O2(g) -> 2HgO(s) + Estrus}\]
\[\text{mercury} + \text{oxygen} \ce{->} \text{mercury(Ii) oxide} + \text{rut}\]
Process
Materials and Equipment
Materials: nine% Hydrogen peroxide solution, manganese(IV) oxide, wooden splints, candle, sulfur, steel wool, magnesium ribbon, zinc metal and 6M hydrochloric acid
Equipment: 250-mL Erlenmeyer flask, five wide-mouth bottles, 4 glass 'encompass' plates, pneumatic trough, "stopper + thistle tube + tubing" apparatus*, utility clamp, stand, deflagration spoon, crucible tongs, modest chalice, medium chalice and a large exam tube.
First, be sure to practise caution when using the hydrogen peroxide (\(\ce{H2O2}\)) and the hydrochloric acid (\(\ce{HCl}\)) as they can cause chemical burns and pare irritation. If either of these chemicals comes into contact with your skin, immediately rinse with water for a minimum of fifteen minutes and notify your instructor. Second, do non await direct at the called-for magnesium. In add-on to existence very brilliant, it emits harmful UV radiation that could cause impairment to the retina of your optics.
Part A: Generating and Collecting Oxygen Gas
- Obtain the following equipment:
- A 250-mL Erlenmeyer flask (locker)
- The "two-hole stopper + thistle tube + glass tubing + rubber tubing" apparatus (stockroom)
- Five broad mouth 'gas-collecting' bottles (under sink)
- Four drinking glass 'encompass' plates (front desk)
- A pneumatic trough (under sink) filled with water to one⁄2 inch above the metal shelf
- Fill four of the five wide-mouth bottles to the skirt with water (the 5th will be used later). And so gently slide a glass plate over the mouth of each bottle. Make certain that in that location are no air bubbles at the peak of the glass plate.
- While holding the glass plate with your fore and middle finger, gently invert a bottle and lower information technology into the water in the pneumatic trough. Remove the glass plate when the oral fissure of the bottle is beneath the water level in the pneumatic trough. Repeat this for all four bottles. Place the glass plates aside on a newspaper towel, every bit they will be used later.
- Place 1 gas-collecting bottle on the metal shelf. Make sure that the oral fissure of the bottle does not come out of the water.
- Now focus on your reaction vessel, the Erlenmeyer flask. Add together a pea-sized amount of manganese(Iv) oxide (the catalyst) to the flask, followed by about 50-mL of tap water.
- Finally, assemble all your equipment together as demonstrated by your teacher, or as shown in the figure below. Make sure that
- the stop of the thistle tube is completely covered with water at the bottom of the flask,
- the stop of the glass tubing running from the Erlenmeyer flask is inserted nether the opening in the
- bottom of the metal shelf into the gas-collecting bottle (which is full of h2o),
- the Erlenmeyer flask is stabilized with a utility clamp.
- Obtain nigh 30-mL of 9% aqueous hydrogen peroxide (\(\ce{H2O2}\)) in your smallest chalice. Then advisedly add about 10-mL of this \(\ce{H2O2}\) through the thistle tube. The generation of oxygen gas should begin immediately. If at any time the rate of the reaction in the Erlemeyer flask appears to slow down, add some other x-mL portion of \(\ce{H2O2}\).
- The oxygen produced will fill the inverted bottle by displacing the water in it. This is because oxygen does not dissolve in water, due to its low solubility. When the starting time bottle is completely filled with gas, identify the second bottle on the metal rack in its place and allow it to fill in a similar manner. Repeat this for the 3rd and fourth bottles.
- As soon equally each bottle is completely filled, remove it by placing a glass plate under the bottle's mouth while under h2o, so lifting the bottle and plate from the pneumatic trough. Identify the bottle on the lab bench mouth up and practice not remove the glass plate. Since oxygen is denser than air, it sinks to the bottom of the flask and will not readily leak out the top.
- Using masking record, label each bottle of gas in the lodge they are nerveless: Bottle #one, Bottle #2, Bottle #3 and Canteen #4. Label the fifth unused empty bottle "Air Bottle".
- In one case all four bottles are filled with oxygen, practise non add any more \(\ce{H2O2}\) to the Erlenmeyer flask. Prepare it bated and allow the reaction to go to completion. At the end of the lab, the chemicals remaining in the reaction flask and any unused \(\ce{H2O2}\) must be disposed of in the labeled waste matter container in the hood. In the meantime, proceed to Part B.
Role B: The Properties of Oxygen Gas
Dispose of all chemicals used in these tests as indicated by your instructor
Test 1: Combustion of wood
Light a wooden split, and so blow information technology out. While it is nevertheless glowing crimson, apace insert the splint into Canteen #1 (oxygen-filled). How many times can you repeat this? Record your observations. Now re-light the aforementioned wooden divide, and over again blow it out. Place information technology in the empty bottle (air-filled) while it is nevertheless glowing. Record your observations.
Test ii: Combustion of candle wax
Place a small candle on a glass plate and light it. Then uncover and carefully lower Bottle #2 (oxygen-filled) over the candle. Measure and record the number of seconds that the candle continues to burn. And then re-light the candle lower the empty bottle (air-filled) over it. Once again, mensurate and tape the number of seconds that the candle continues to burn. Also be sure to record any other relevant observations.
Examination 3: Combustion of sulfur
This test must be performed in the hood under instructor supervision. Take your Canteen #3 (oxygen-filled) and your empty bottle (air-filled) to the hood your instructor directs you lot to. Place a pocket-sized lump of sulfur in a deflagrating spoon (located in the hood). Light the Bunsen burner in the hood, and rut the sulfur in the spoon. The sulfur will first melt, then fire with an almost invisible blue flame. Insert the spoon with the burning sulfur in Bottle #3 and tape your observations. Then insert information technology in the empty bottle, and again record your observations. When finished, extinguish the burning sulfur in the beaker of water provided in the hood.
Examination iv: Combustion of fe
Pour most 20-mL of tap water into Bottle #4 (oxygen-filled) and replace the glass plate apace. Have a loose, frayed out ii-iii centimeter piece of steel wool and agree information technology in a Bunsen burner flame for a very brief instant with your crucible tongs (information technology will glow red). Then immediately lower the steel wool into Bottle #4. Tape your observations. Repeat with the empty bottle (air-filled) and tape your observations.
Test v: Combustion of hydrogen
This examination must be performed in air only. (Note: The hydrogen burned in this test must be start generated by a reaction betwixt zinc and hydrochloric acid.) To a large test tube, add i-2 pieces of zinc metal followed by virtually 3-mL of muriatic acid. Rapid bubbling should begin immediately as hydrogen gas is produced, and the lesser of the examination tube volition get quite hot. Identify the exam tube in the medium chalice. Afterwards 60 seconds take elapsed, calorie-free a wooden splint. Do not blow information technology out. Concord the burning splint to the oral cavity of test tube (where the hydrogen gas is being evolved) and record your observations.
Test 6: Combustion of magnesium
This exam is an instructor demonstration. It must be performed in air only. Hold a 1-inch slice of magnesium metal in a Bunsen burner flame with your crucible tongs until information technology ignites (in air). Record your observations, remembering non to await direct at the burning magnesium!
Pre-laboratory Assignment: The Properties of Oxygen Gas
- Oxygen gas will produced via a decomposition reaction of a certain substance.
- Name the substance that will exist decomposed.
- Name the two products generated by this reaction.
- A goad chosen manganese(IV) oxide, \(\ce{MnO2}\), will exist used to facilitate the production of oxygen gas. Exactly what does the catalyst practise?
- Carefully read the procedure for producing oxygen gas (Part A) and examine the accompanying figure of the equipment set-upwardly
- What type of flask does the decomposition reaction occur in?
- What chemical is added to this flask through the thistle tube?
- What chemic(south) are already in the flask?
- What type of bottles is the oxygen gas collected in?
- After the oxygen is collected, exercise you shop it in these bottles right-side up or upside down? (circle i) Explain why.
- After generating and collecting the oxygen, you will and then investigate its function in combustion reactions
- Is oxygen a reactant or product in a combustion reaction?
- Are combustion reactions exothermic or endothermic?
- In Office B you will burn a variety of substances in the oxygen gas collected from Part A.
- Which 1 of these substances must be burned in the hood?
- Which two of these substances must be burned in air simply?
- Which substance (i only) will be burned by the teacher?
Lab Written report: The Backdrop of Oxygen Gas
Role A: Generating and Collecting Oxygen Gas
- Write the equation for the reaction used to generate oxygen gas.
- Give-and-take Equation:
- Formula Equation:
- What is the name and formula of the catalyst used in this reaction? What is the purpose of this catalyst?
- In addition to oxygen, what other substance is produced past this reaction? Where is this substance collected?
- Two notable physical properties of oxygen are its low solubility in water and a density greater than air
- Which one of these properties allows the oxygen gas collected to be stored in the bottles mouth upwards? Explain.
- Which one of these properties allows the oxygen gas to be nerveless via the displacement of h2o? Explain.
Role B: The Properties of Oxygen Gas
| Test | Observations |
|---|---|
| Test 1 | |
| Glowing splint in Bottle #1 | |
| Glowing splint in air bottle |
| Exam 2 | |
| Burning candle in Bottle #two | Candle burned for _______ seconds. |
| Called-for candle in air bottle | Candle burned for _______ seconds. |
| Test 3 | |
| Burning sulfur in Bottle #3 | |
| Burning sulfur in air bottle | |
| Test 4 | |
| Glowing steel in Bottle #4 | |
| Glowing steel in air bottle | |
| Test 5 | |
| Called-for hydrogen in air | |
| Exam 6 | |
| Burning magnesium in air |
Analysis of Combustion Results
- Consider your results for the first 4 tests yous performed. In which bottles, air-filled or oxygen-filled, did the combustion reactions occur more than vigorously? Why?
- Are the combustion reactions of oxygen exothermic or endothermic? Support your answer with one or more specific observations from the tests y'all performed.
- Consider your Examination 2 results. Although the candle burns for a longer period of time in i bottle, it eventually goes out in both the empty bottle and Bottle #two. Why does it get out?
- When an element burns in oxygen gas, the product is chosen an oxide.
- The wood in the splint consists mostly of carbon. The combustion of carbon produces carbon dioxide, \(\ce{CO2}\). Write the equation for the combustion of woods (carbon).
- Word Equation:
- Counterbalanced Formula Equation:
- The combustion of sulfur produces sulfur dioxide, \(\ce{SO2}\). Write the equation for the combustion of sulfur.
- Word Equation:
- Balanced Formula Equation:
- Steel wool consists mostly of iron. The combustion of atomic number 26 produces atomic number 26(III) oxide, \(\ce{Fe2O3}\). Write the equation for the combustion of steel wool (iron).
- Word Equation:
- Balanced Formula Equation:
- The combustion of hydrogen produces water, \(\ce{H2O}\). Write the equation for the combustion of hydrogen.
- Give-and-take Equation:
- Balanced Formula Equation:
- The combustion of magnesium produces magnesium oxide, \(\ce{MgO}\). Write the equation for the combustion of magnesium.
- Word Equation:
- Balanced Formula Equation:
- Practise you expect the product formed during the combustion of magnesium in Test half-dozen (the ashy magnesium oxide) to counterbalance more than than, less than, or the same as the original slice of magnesium? Explain.
Source: https://chem.libretexts.org/Ancillary_Materials/Laboratory_Experiments/Wet_Lab_Experiments/General_Chemistry_Labs/Online_Chemistry_Lab_Manual/Chem_10_Experiments/04%3A_The_Properties_of_Oxygen_Gas_(Experiment)
0 Response to "what happened when the splint was brought to the mouth of the first bottle of gas collected"
ارسال یک نظر